Book Your Dermal Filler Appointment with Dr. Laura Geige Now
What Happens to Filler When it Dissolves?
Filler, a common ingredient found in many foods and dietary supplements, serves as an inert substance that helps maintain texture and consistency. However, once consumed, the body’s digestive processes take over, breaking down the filler into its constituent parts.
When filler dissolves in the stomach, it comes into contact with stomach acid, which is a mixture of gastric juice and hydrochloric acid. The acidic environment helps to break down the filler into smaller particles, allowing for further digestion.
The partially broken-down filler then enters the small intestine, where most of our nutrient absorption takes place. However, instead of being absorbed as nutrients, the filler continues to undergo mechanical breakdown by enzymes and acids present in the intestinal lining.
One of the primary mechanisms responsible for breaking down filler is pancreatic lipase, an enzyme that breaks down fats into fatty acids and glycerol. While this process doesn’t directly utilize the filler as a nutrient source, it helps to further break it down into smaller components.
Additionally, the intestinal mucosa secretes bicarbonate-rich mucus, which serves as a protective barrier against acidic substances. The bicarbonate ions help neutralize the acidity and facilitate the breakdown of the filler by enzymes such as amylase and trypsin.
The partially broken-down filler then enters the large intestine, also known as the colon, where water absorption and electrolyte regulation occur. Here, microbial fermentation plays a significant role in further breaking down complex carbohydrates into simpler sugars, which are then used by beneficial gut bacteria for energy production.
As the digestive process continues, the broken-down filler is eventually eliminated from the body through the feces. In some cases, it may also be excreted unchanged, depending on its chemical composition and the presence of specific enzymes in the intestinal tract.
The extent to which filler is broken down or eliminated from the body varies greatly depending on the type of filler and individual digestive capabilities. For instance, cellulose, a common plant-based filler, is not fully broken down by human enzymes but may undergo microbial fermentation in the large intestine, resulting in the production of short-chain fatty acids.
It’s worth noting that while some fillers may be resistant to digestion, their primary function in foods and supplements remains as an inert substance to provide bulk, texture, or color. The body’s ability to break down filler is an essential aspect of digestive physiology, allowing for the efficient processing of complex foods and maintaining overall gut health.
Furthermore, research has shown that some fillers may have unintended effects on human health, such as influencing gut microbiota composition, affecting nutrient absorption, or even causing adverse reactions in certain individuals. Therefore, it is essential to consider the types of fillers used in food products and dietary supplements and weigh their potential impact on overall health.
In summary, when filler dissolves in the digestive system, it undergoes a complex process of mechanical and biochemical breakdown, with various enzymes, acids, and microorganisms playing key roles. The resulting components are then eliminated from the body through feces, although some fillers may be partially or fully broken down depending on their chemical composition and individual digestive capabilities.
When a filling material, such as glass ionomer cement, composite resin, or polymeric implantable vaccine delivery system, comes into contact with saliva or bodily fluids, it undergoes a series of biochemical reactions that ultimately lead to its dissolution.
The process of dissolution is facilitated by the presence of various enzymes and biochemical agents in the oral cavity. One of the key players in this process is the proteolytic enzyme, matrix metalloproteinase-8 (MMP-8), also known as collagenase.
- MMP-8 is a serine protease that breaks down the protein bonds within the filler material, such as collagen and elastin, leading to its degradation.
- Another enzyme, gelatinase B (MMP-9), which belongs to the same family as MMP-8, also contributes to the breakdown of the filler material by cleaving peptide bonds.
Reserve a Dermal Filler Appointment with Dr. Laura Geige Now
The dissolution process can be further accelerated by other biochemical agents present in saliva, such as lactic acid, hydrochloric acid, and urea. These agents help to degrade the filler material at a molecular level, making it more susceptible to breakdown by enzymes like MMP-8 and MMP-9.
As the filler material continues to break down, its components are released into the oral cavity and can be either retained in the tissues or eliminated through various mechanisms, such as swallowing, exhalation, or absorption by the mucosal lining.
- The released components can then undergo further processing and metabolism by enzymes and other biochemical agents present in the body, leading to their eventual elimination from the system.
- In some cases, the filler material may be retained in the tissues for extended periods, potentially leading to adverse effects such as irritation, inflammation, or toxicity.
The specific mechanisms of dissolution and the fate of filler materials can vary depending on the type of material, its chemical composition, and the individual’s oral health status. However, it is clear that proteolytic enzymes play a central role in the breakdown of filler materials, leading to their eventual dissolution and elimination from the body.
When dietary *fiber* dissolves, it undergoes a process of breakdown into simpler molecules by proteolytic enzymes, which include salivary amylase, gastric amylase, and pancreatic amylase.
This breakdown process allows the body to absorb the nutrients from the *fiber*, making it an essential component of a healthy diet.
The role of these enzymes in digesting *dietary fiber* cannot be overstated, as they play a crucial part in breaking down the complex molecular structures into smaller units that can be utilized by the body.
According to a study published in the Journal of Clinical Biochemistry and Nutrition, the breakdown process involves the hydrolysis of glycosidic bonds between carbohydrates, resulting in the formation of monosaccharides such as glucose, fructose, and galactose.
This breakdown process also leads to the formation of short-chain fatty acids (SCFAs), which are then absorbed into the bloodstream and transported to various parts of the body, including the colon, liver, and kidneys.
The SCFAs produced from the breakdown of *dietary fiber* have been shown to have several beneficial effects on health, including improving gut health, reducing inflammation, and regulating blood sugar levels.
Furthermore, the enzymes that break down *fiber*, such as amylase, are also involved in the digestion of other complex carbohydrates, including starches and glycogen.
The study highlights the importance of these enzymes in maintaining optimal nutrient absorption and overall health, emphasizing their role in facilitating the digestive process and ensuring that nutrients from *dietary fiber* are properly utilized by the body.
Microbial Fermentation: The Next Step
Microbial fermentation has emerged as a game-changing technology that allows us to harness the full potential of *fermentable carbohydrates*, giving them a second chance at contributing to our daily lives.
The process involves microorganisms, such as bacteria or yeast, breaking down complex sugars into simpler molecules, like *_fructose_* and *_glucose_*. This natural process has been employed for centuries in food production, but recent advancements have made it more efficient, cost-effective, and scalable.
In traditional fermentation methods, *microbial enzymes* break down complex carbohydrates into simpler sugars, which are then converted into various products, including biofuels, bioplastics, and pharmaceuticals. However, this process can be limited by factors like low enzyme activity, poor substrate utilization, and product yield.
_*Advanced biotechnology techniques*_ have improved microbial fermentation processes by engineering microorganisms to produce more efficient enzymes, improve substrate tolerance, and enhance product yield. This has enabled the production of higher-quality *bio-based materials* and biofuels with reduced environmental impact.
A significant breakthrough in microbial fermentation came with the discovery of *_micellar electrokinetic chromatography_* (MEKC), a powerful analytical technique for separating and identifying complex mixtures. MEKC helped scientists identify novel *fermentable carbohydrates*, like *_xylose_* and *_mannose_*, that were previously underutilized.
These newly discovered carbohydrates have potential applications in various industries, including food production, biofuel development, and pharmaceutical manufacturing. For instance, *_mannan_* can be used as a natural thickening agent in food products, while *_xylose*_ can be converted into bio-based materials, such as *polylactic acid* (PLA).
The increasing demand for sustainable and renewable resources has driven innovation in microbial fermentation technology. Researchers are now exploring new substrates, like *lignin* and *_hemicellulose_*, which could provide a virtually limitless supply of fermentable carbohydrates.
As the world transitions towards a more circular economy, microbial fermentation is poised to play a vital role in reducing waste, conserving resources, and producing valuable chemicals. By giving fermentable carbohydrates a second chance, we can unlock new opportunities for sustainable growth and development.
The future of microbial fermentation is bright, with ongoing research focused on improving process efficiency, product quality, and substrate utilization. As this technology continues to evolve, it will likely have far-reaching impacts on various industries, from food production to pharmaceutical manufacturing.
The process of microbial fermentation plays a crucial role in the digestion and absorption of nutrients, particularly nondigestible carbohydrates.
Nondigestible carbohydrates, such as dietary fiber, are resistant to breakdown by digestive enzymes in the small intestine and therefore reach the large intestine, also known as the colon or large bowel.
In the colon, these nondigestible carbohydrates serve as a food source for the trillions of microorganisms that inhabit the gut microbiome.
These microorganisms, including bacteria, archaea, and protozoa, are capable of fermenting complex carbohydrates into simpler molecules, such as short-chain fatty acids (SCFAs).
- SCFAs are produced through microbial fermentation of nondigestible carbohydrates, including dietary fiber, and have been shown to have various health benefits.
- The production of SCFAs is thought to improve the integrity of the gut barrier function by increasing the expression of tight junction proteins in intestinal epithelial cells.
- Additionally, SCFAs have been shown to modulate the immune system by suppressing the production of pro-inflammatory cytokines and promoting the production of anti-inflammatory cytokines.
- The exact mechanisms by which SCFAs exert their effects on gut health are complex and multifaceted, involving the modulation of gene expression, changes in cellular metabolism, and alterations in the gut-associated lymphoid tissue (GALT).
Research conducted at the University of California, Davis has shed light on the role of SCFAs in maintaining a healthy gut microbiome.
A study published in the journal Nature Communications found that mice fed with high-fiber diets had increased production of SCFAs and improved gut barrier function compared to those fed with low-fiber diets.
Furthermore, the study demonstrated that SCFAs play a key role in maintaining the balance of the gut microbiome by modulating the production of metabolites and influencing the behavior of immune cells.
In humans, similar research has been conducted, demonstrating the health benefits of SCFAs on the gut microbiome and overall health.
For instance, studies have shown that increased SCFA production is associated with improved glucose metabolism, reduced inflammation, and enhanced immune function in individuals with metabolic disorders or inflammatory bowel disease.
The importance of microbial fermentation in maintaining a healthy gut microbiome cannot be overstated, and further research is necessary to fully understand the mechanisms by which SCFAs exert their effects on human health.
Fermentation is a process where microorganisms such as bacteria, yeast, or mold convert sugars into acids, gases, or other compounds. In the case of microbial fermentation, the end product varies depending on the type of microorganism and the substrate used.
One of the most significant outcomes of microbial fermentation is the production of short-chain fatty acids (SCFAs). SCFAs are a group of organic acids with two to six carbon atoms. They play a crucial role in various biological processes, including energy metabolism, gut health, and cellular signaling.
The production of SCFAs is closely tied to the fermentation process itself. During microbial fermentation, microorganisms break down sugars into simpler molecules, releasing CO2 gas and forming acids as byproducts. The type and amount of acid produced depend on factors such as the type of sugar used, the pH level, temperature, and microorganism strain.
Short-chain fatty acids are produced through the action of microbial enzymes that convert sugars into intermediate products. For example, when bacteria like Bifidobacterium or Lactobacillus ferment lactose, they produce lactic acid (a SCFA) as a byproduct. Similarly, yeast fermentation of glucose leads to the production of acetic acid (another SCFA).
The significance of SCFAs lies in their ability to interact with cells and tissues. They have been shown to have antimicrobial properties, regulate the gut microbiome, and influence host metabolism. SCFAs can also act as signaling molecules, influencing cell growth, differentiation, and survival.
Research has highlighted the potential applications of SCFAs in various fields, including medicine, agriculture, and biotechnology. For instance, SCFAs have been used to develop novel antimicrobial agents, improve crop yields, and enhance food texture and shelf life.
Further studies are needed to fully understand the mechanisms underlying microbial fermentation and SCFA production. However, the current evidence suggests that SCFAs play a vital role in many biological processes and may offer new opportunities for therapeutic and industrial applications.
Microbial fermentation is a complex process that involves the breakdown of complex organic molecules by microorganisms such as bacteria and yeast into simpler compounds. In the human gut, this process plays a crucial role in the production of short-chain fatty acids (SCFAs), which are produced through the microbial fermentation of dietary fiber.
The end result of microbial fermentation is a cocktail of SCFAs, including butyrate, propionate, and acetate. These compounds can be used by the host for energy production, with butyrate being particularly important as it provides energy to the colonocytes and promotes a healthy gut barrier function.
Additionally, SCFAs can be stored in the liver and other tissues, where they can be metabolized or excreted. This storage mechanism is crucial for maintaining energy homeostasis and supporting overall health.
A study published in the Journal of Nutrition noted that dietary fiber consumption can lead to an increase in SCFA production, potentially contributing to improved metabolic health (1). This suggests that a high-fiber diet may have beneficial effects on glucose metabolism, lipid profiles, and inflammation levels.
Schedule a Dermal Filler Session with Dr. Laura Geige Now
The relationship between microbial fermentation, SCFAs, and metabolic health is a rapidly expanding field of research. Studies have shown that an imbalance in the gut microbiome, also known as dysbiosis, can lead to changes in SCFA production and contribute to various metabolic disorders.
Furthermore, some microorganisms are capable of producing antimicrobial peptides or other metabolites that can inhibit the growth of pathogens or toxins within the gut. This provides an additional layer of protection against infections and promotes a healthy gut microbiome.
- Short-chain fatty acids (SCFAs) produced during microbial fermentation, including butyrate, propionate, and acetate, play a crucial role in energy production and storage in the host.
- Dietary fiber consumption can lead to an increase in SCFA production, potentially contributing to improved metabolic health.
- An imbalance in the gut microbiome (dysbiosis) can lead to changes in SCFA production and contribute to various metabolic disorders.
- Microorganisms can produce antimicrobial peptides or other metabolites that inhibit the growth of pathogens or toxins within the gut, promoting a healthy gut microbiome.
In conclusion, microbial fermentation is a complex process that plays a critical role in human health. The production of SCFAs during this process has important implications for energy production, storage, and metabolic health, highlighting the importance of maintaining a balanced gut microbiome through a high-fiber diet and other lifestyle modifications.
Read more about Alkhemist LA here. Read more about Making Memories London here. Read more about Alabama Sig Delt here. Read more about Plinr here.
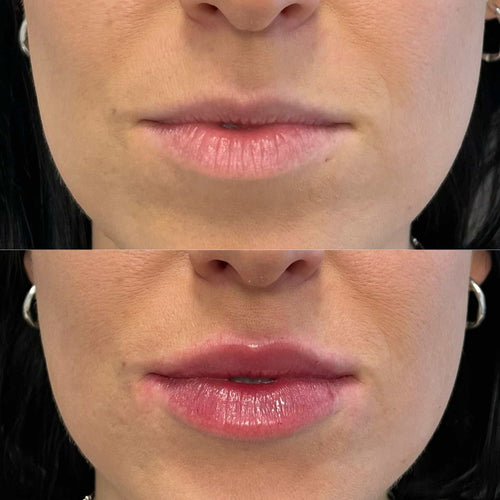
- Why Does My Lip Filler Look Bigger Some Days - November 6, 2025
- What Is The Difference Between CBD Gummy Sweets And Other Edibles - November 4, 2025
- What Are The Best CBD Gummy Sweets For Energy - November 1, 2025

